The WHO may list aspartame, a common artificial sweetener, as potentially carcinogenic. Let us understand the potential hazards of aspartame and the guidelines provided by WHO and the US Food and Drug Administration (FDA)
The World Health Organization's (WHO) cancer research arm could list Aspartame, one of world's most common artificial sweeteners that is commonly used in diet sodas, as potentially carcinogenic (causing cancer) to humans. According to reports, the International Agency for Research on Cancer (IARC) could recommend Aspartame's listing as early as July.

According to a report published in Reuters, following a recent gathering of external experts, the IARC has reached a conclusive decision regarding the potential hazards of a particular substance. The IARC's objective is to evaluate whether a substance poses a potential threat based on a comprehensive analysis of all available published evidence.
It is important to note that the IARC's ruling does not factor in the specific quantity of a product that can be safely consumed by individuals. This aspect of guidance, pertaining to safe consumption levels, is addressed by a separate expert committee of the World Health Organization (WHO) known as JECFA (Joint WHO and Food and Agriculture Organization's Expert Committee on Food Additives).
What is Aspartame?
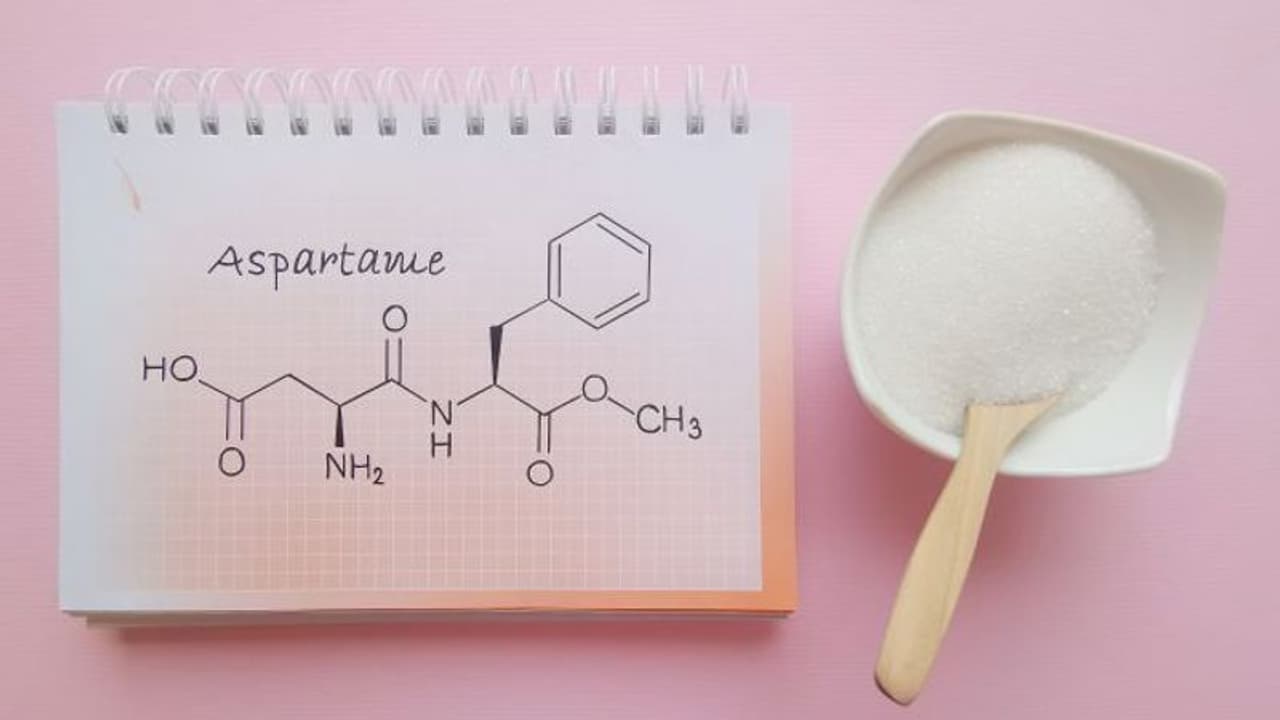
Aspartame is a dipeptide composed primarily of two amino acids: aspartic acid and phenylalanine. These amino acids, along with others, are naturally present in protein-rich foods that are part of a healthy diet.
While aspartame does contain calories, consumers generally tend to use less of it compared to table sugar due to its significantly higher sweetness, which is approximately 200 times sweeter.
When phenylalanine and aspartic acid are combined in a specific manner to create aspartame, it results in a substance with an intensely sweet taste. It's important to note that aspartame is not heat stable and loses its sweetness when exposed to heat, which is why it is typically not used in baked goods.
The US Food and Drug Administration (FDA) regulates aspartame as a food additive. The FDA initially established regulations for aspartame in 1974, allowing its use as a tabletop sweetener and in products such as chewing gum, cold breakfast cereals, and dry bases for certain foods (e.g., beverages, instant coffee and puddings and fillings, dairy products, tea, gelatins and toppings).
Over time, the FDA granted additional approvals for aspartame, including its use as a general-purpose sweetener, which was most recently approved in 1996.
WHO's May Guideline on the Use of Sweeteners
Citing recent research, the World Health Organization (WHO) advised against the use of non-sugar sweeteners (NSS) like aspartame as a means of controlling body weight or reducing the risk of non-communicable diseases (NCDs).
The guideline stems from a comprehensive analysis of available evidence, which indicates that the long-term consumption of NSS does not offer significant benefits in terms of reducing body fat in adults or children.
Furthermore, the review suggests that prolonged use of NSS may potentially lead to undesirable effects, including increased susceptibility to type 2 diabetes, cardiovascular diseases, and mortality in adults.
Francesco Branca, WHO Director for Nutrition and Food Safety, highlighted that substituting free sugars with NSS is not an effective approach for long-term weight management. Instead, individuals are encouraged to explore alternative methods of reducing their intake of free sugars, such as opting for foods containing naturally occurring sugars, like fruits, or consuming unsweetened food and beverages.