On Saturday, the company reported promising early outcomes from its Phase 1b PIONEER trial for sickle cell disease (SCD).
- Piper Sandler boosted its target price to $23 per share from $16.
- Wainwright increased its price target to $25 from $18, noting a clear dose-response in fetal hemoglobin (HbF) and a strong tolerability profile.
- At Week 6, the mean absolute fetal hemoglobin (HbF) rose from a baseline of 7.1% to 16.9%, with 58% of participants reaching HbF levels of 20% or more.
Fulcrum Therapeutics (FULC) is on track to reach its March 2024 highs after it received Wall Street’s backing following encouraging early data from its sickle‑cell disease drug candidate.

On Saturday, the company reported promising early outcomes from its Phase 1b PIONEER trial for sickle cell disease (SCD).
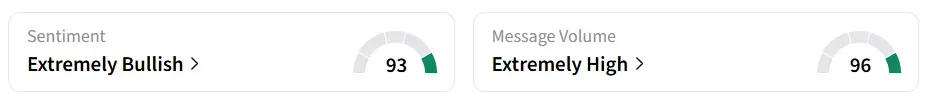
Fulcrum Therapeutics’ stock traded over 51% higher in Monday’s premarket. On Stocktwits, retail sentiment around the stock remained in ‘extremely bullish’ territory amid ‘extremely high’ message volumes.
Analyst Backing Grows After Positive Trial Signals
Piper Sandler boosted its target price to $23 per share from $16 while retaining an Overweight rating. H.C. Wainwright also increased its price target to $25 from $18 and kept a Buy rating.
Wainwright pointed to topline results from the 20 mg cohort of Fulcrum’s Phase 1b PIONEER trial, noting a clear dose-response in fetal hemoglobin (HbF) and a strong tolerability profile, which together bolster hopes for the drug’s potential in sickle‑cell disease. The shifts in analyst sentiment come as Fulcrum prepares to release full 12‑week data in the first quarter of 2026.
Study Findings
In the study, 12 adult participants with severe SCD were evaluated, with Week 6 results available for all participants and Week 12 results available for the first 6.
At Week 6, the mean absolute fetal hemoglobin (HbF) rose from a baseline of 7.1% to 16.9%, with 58% of participants reaching HbF levels of 20% or more, a threshold associated with markedly fewer vaso-occlusive crises (VOCs). The Week 12 analysis of the first six patients showed more than a 3.75-fold increase in HbF induction, highlighting a clear dose-response compared with prior 12 mg cohorts.
For updates and corrections, email newsroom[at]stocktwits[dot]com.<
