The U.S. Food and Drug Administration cleared its Investigational New Drug application for SER-252, a therapy to treat patients with Parkinson’s disease.
- The clearance allows the clinical-stage biotech firm to move ahead with preparations for a planned Phase 1b registrational study.
- Serina had previously received written feedback from the agency supporting its proposed registrational trial design under an NDA pathway.
- The latest update follows a setback in November 2025, when the FDA placed a clinical hold on the application to initiate clinical trials.
Shares of Serina Therapeutics (SER) surged more than 70% in pre-market trading on Thursday after the company announced that the U.S. Food and Drug Administration (FDA) cleared its Investigational New Drug (IND) application for SER-252, a therapy to treat patients with Parkinson’s disease.

SER-252 is an experimental apomorphine-based therapy to provide continuous dopaminergic stimulation, an approach shown to ease levodopa-related motor complications such as dyskinesia in people with Parkinson’s disease.
SER stock had surged nearly 35% in after-hours trading on Wednesday.
SER-252: What Next?
The clearance allows the clinical-stage biotech firm to move ahead with preparations for a planned Phase 1b registrational study in patients with advanced Parkinson’s disease. The IND clearance follows extensive regulatory engagement between Serina and the FDA.
The company previously received written feedback from the agency supporting its proposed registrational trial design under an NDA pathway.
“With FDA alignment on a registrational development strategy under a 505(b)(2) NDA pathway, we believe SER-252 has a clear and efficient path forward toward addressing a significant unmet medical need,” said Steve Ledger, Chief Executive Officer of Serina Therapeutics.
The latest update follows a setback in November 2025, when the FDA placed a clinical hold on Serina’s application to initiate clinical trials. That hold was tied to a request for additional information related to an excipient in the drug’s formulation, a non-active substance included in a drug formulation alongside the active ingredient. Serina emphasized that the FDA’s concerns did not involve SER-252’s active drug substance or its proposed mechanism of action.
Retail Reaction
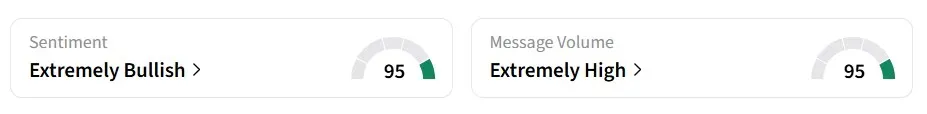
Retail sentiment on Stocktwits turned ‘extremely bullish’ from ‘bearish’ a day earlier, accompanied by ‘extremely high’ message volumes.
One bullish user said the stock price could double with good volumes.
Another user simply said “massive news”, and the stock could climb to $8. It is currently around $4.
SER shares have shed more than 40% in the past year, though its year-to-date gains are over 25%.
Read also: Nasdaq, S&P 500 Futures Climb Ahead Of Apple Earnings: Why META, TSLA, MP, CRML, SER Are On Traders' Radar Today
For updates and corrections, email newsroom[at]stocktwits[dot]com<