Living cells may generate electricity through tiny membrane movements, scientists suggest. Molecular activity can create nerve-like voltage spikes, helping drive ion transport and inspiring bio inspired materials.
Living cells may be capable of generating their own electricity using nothing more than microscopic motion, according to a new scientific framework. Researchers suggest that constant movement within cell membranes can create electrical signals similar to those used by nerve cells, offering a new way to understand how life generates and uses energy. The findings are published in the journal PNAS Nexus.

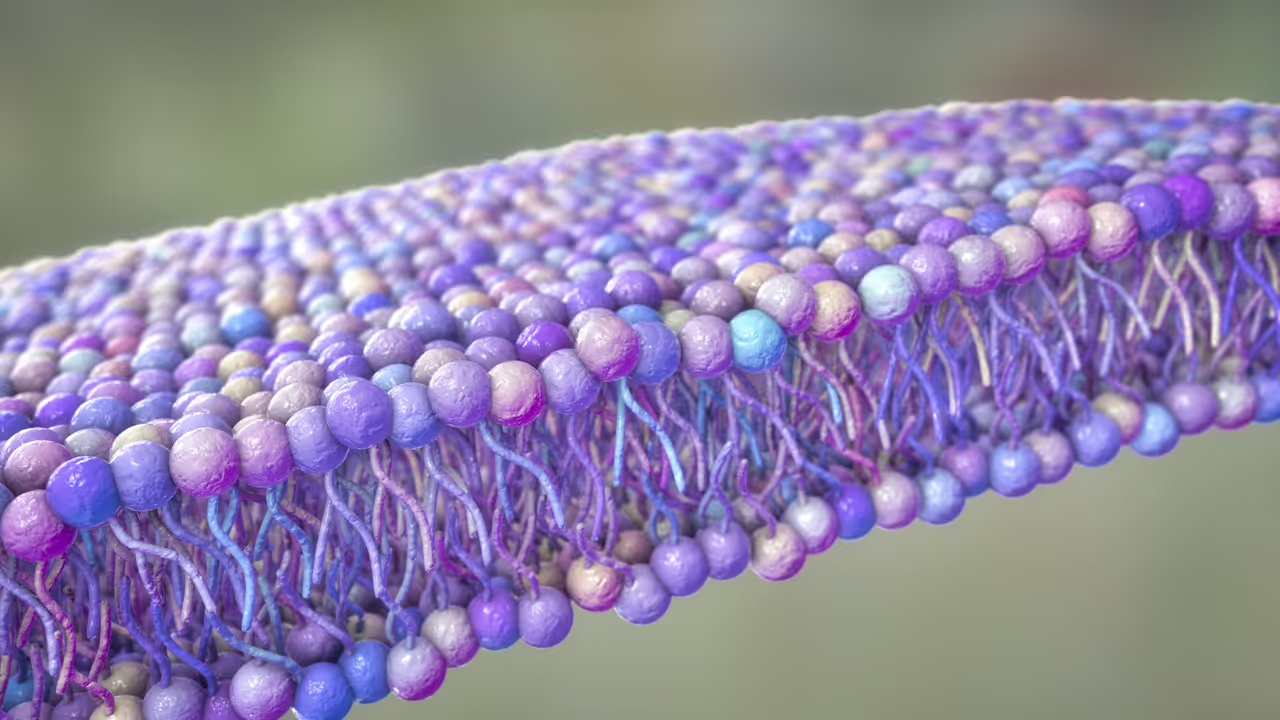
Rather than acting as a passive barrier, the cell membrane emerges as an active, dynamic structure that converts motion into electrical activity.
How Tiny Movements Create Electrical Signals
Every cell is wrapped in a thin, flexible membrane that constantly bends, ripples, and reshapes itself. These movements are driven by active molecular processes inside the cell, particularly proteins that consume energy through ATP breakdown.
As these proteins change shape and interact with surrounding molecules, they exert mechanical forces on the membrane. The new model shows that this mechanical activity produces “active noise” tiny fluctuations that cause the membrane to bend outward and inward.
This bending triggers a physical effect known as flexoelectricity, where mechanical deformation generates an electrical voltage. In simple terms, when the membrane bends, it can create an electrical difference between the inside and outside of the cell.
Voltages Comparable to Neuron Signals
Surprisingly, the model predicts that these motion-driven voltages can be quite strong. In some cases, they may reach up to 90 millivolts, which is comparable to the electrical signals produced by neurons during nerve firing.
The speed of these voltage changes is also striking. The electrical spikes occur within milliseconds, closely resembling the timing and shape of neuronal action potentials. This suggests that basic physical processes in cell membranes may contribute to how nerve cells transmit information.
Researchers believe this mechanism could help explain electrical behavior in cells that do not traditionally generate action potentials, expanding the understanding of bioelectricity beyond the nervous system.
Powering Ion Transport and Future Technologies
The framework also suggests that membrane-generated voltages could actively move ions across the membrane. Ions are essential for cellular communication and balance, and they typically move along natural concentration gradients.
According to the model, membrane motion could push ions against these gradients, providing a previously unrecognized source of energy for active transport. The direction and strength of ion movement depend on the membrane’s physical properties, including its elasticity and electrical responsiveness.
Beyond biology, the findings may influence future technology. The same principles could be applied to tissues made of many cells, helping scientists understand large scale electrical patterns in living systems.
Researchers also see potential applications in designing bio-inspired materials smart systems that generate electricity from motion, much like living cells do. This work may help bridge the gap between biology, physics, and the development of intelligent materials that mimic life itself.