Vaccine for Coronavirus: Sputnik V to land in Kanpur for human trial
The first batch of Russia's Sputnik V vaccine for Coronavirus is likely to reach next week at Kanpur's Ganesh Shankar Vidyarthi Medical College where the vaccine's Phase 2 and Phase 3 human clinical trials will be conducted.
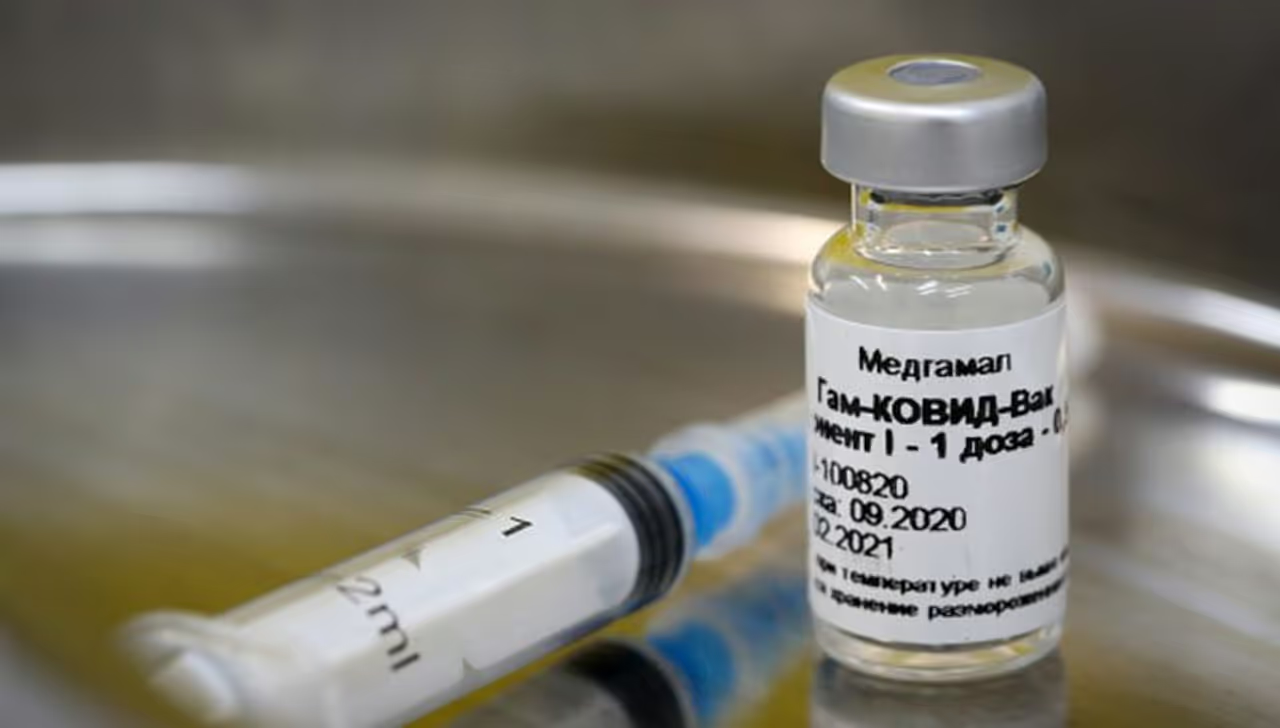
<p>The first batch of Russia's Sputnik V vaccine for Coronavirus is likely to reach next week at Kanpur's Ganesh Shankar Vidyarthi Medical College where the vaccine's Phase 2 and Phase 3 human clinical trials will be conducted.</p>
The first batch of Russia's Sputnik V vaccine for Coronavirus is likely to reach next week at Kanpur's Ganesh Shankar Vidyarthi Medical College where the vaccine's Phase 2 and Phase 3 human clinical trials will be conducted.

<p>The decision to conduct the human clinical trials of the vaccine was taken after Dr Reddy’s Laboratories got approval from the Drugs Controller General of India in this regard.</p>
The decision to conduct the human clinical trials of the vaccine was taken after Dr Reddy’s Laboratories got approval from the Drugs Controller General of India in this regard.
<p>"As many as 180 volunteers have registered for the trials. Head of the research Saurabh Agarwal will determine the dosage of the vaccine to be administered. One dose will be administered and the condition of volunteers will be monitored to determine whether they need further doses or not," college principal R B Kamal told news agency<em> PTI</em>.</p>
"As many as 180 volunteers have registered for the trials. Head of the research Saurabh Agarwal will determine the dosage of the vaccine to be administered. One dose will be administered and the condition of volunteers will be monitored to determine whether they need further doses or not," college principal R B Kamal told news agency PTI.
<p>The Sputnik V vaccine is based on a well-studied human adenoviral vector platform that had proven safe and effective with no long-term side effects in more than 250 clinical trials globally conducted during the past two decades (while the history of use of human adenoviruses in vaccine development started in 1953). </p>
The Sputnik V vaccine is based on a well-studied human adenoviral vector platform that had proven safe and effective with no long-term side effects in more than 250 clinical trials globally conducted during the past two decades (while the history of use of human adenoviruses in vaccine development started in 1953).
<p>More than 100,000 people have received approved and registered drugs based on the human adenoviral vectors. The uniqueness of the Russian vaccine is in using two different human adenoviral vectors that enable to provide strong and long-term immune response after the second injection.</p>
More than 100,000 people have received approved and registered drugs based on the human adenoviral vectors. The uniqueness of the Russian vaccine is in using two different human adenoviral vectors that enable to provide strong and long-term immune response after the second injection.
<p>The Sputnik V vaccine efficacy amounted to 92% (calculation based on the 20 confirmed COVID-19 cases split between vaccinated individuals and those who received the placebo). </p>
The Sputnik V vaccine efficacy amounted to 92% (calculation based on the 20 confirmed COVID-19 cases split between vaccinated individuals and those who received the placebo).
<p>Currently 40,000 volunteers are taking part in double-blind, randomized, placebo-controlled Phase III of Sputnik V clinical trials, out of which over 20,000 have been vaccinated with the first dose of the vaccine and more than 16,000 with both the first and second doses of the vaccine.</p>
Currently 40,000 volunteers are taking part in double-blind, randomized, placebo-controlled Phase III of Sputnik V clinical trials, out of which over 20,000 have been vaccinated with the first dose of the vaccine and more than 16,000 with both the first and second doses of the vaccine.