The PwC India-ISAIC report noted that in addition to the regulatory reforms, India is setting up various collaborative centres for research and development across diseases of high burden. Collaborating with these centres can enable faster access to sites and patients for the top biopharma for diseases with high unmet need
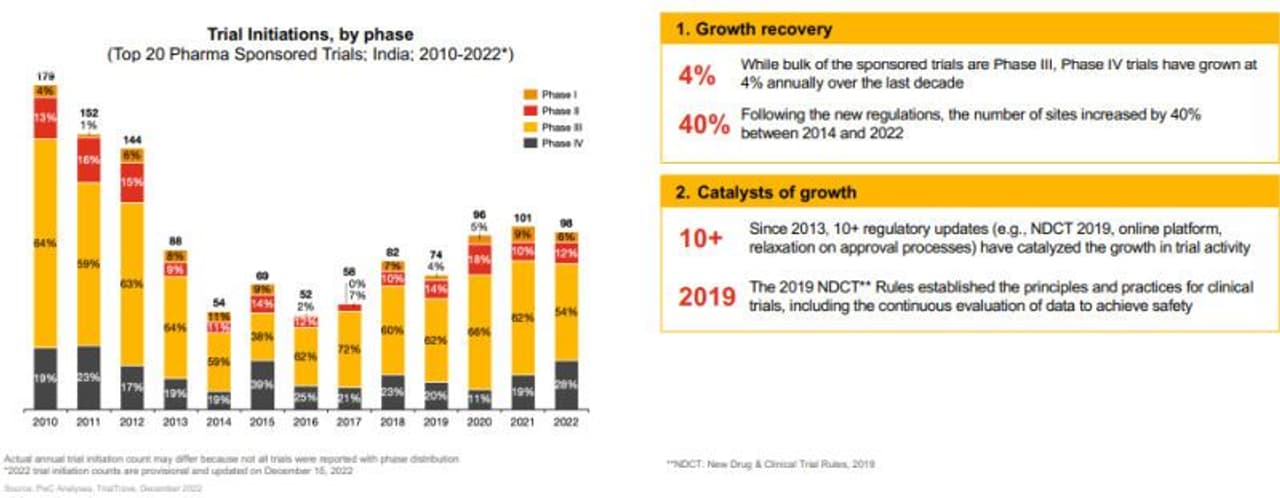
Clinical trial activity in India was historically low until 2014 due to non-favourable regulations but since 2014, the country has witnessed a reversal of historical decline, a joint report by PwC India and USAIC (US-India Chamber of Commerce) titled 'Clinical Trial opportunities in India' has said.

The report, which was released at the USAIC BioPharma & Healthcare Summit held virtually on May 3, India is emerging as a favourable destination to conduct clinical trials through several key drivers. It notes that given the current geopolitical environment, trial sponsors can consider India as an enabler of innovation.
The PwC India-ISAIC report noted that in addition to the regulatory reforms, India is setting up various collaborative centres for research and development across diseases of high burden. Collaborating with these centres can enable faster access to sites and patients for the top biopharma for diseases with high unmet needs.
Sujay Shetty, Partner & Global Health Industries Leader, PwC, said, "Clinical trial activity in India has been increasing steadily since 2014 due to several key regulatory reforms aimed towards global harmonisation, enabling open access to clinical trials in India. The country’s diverse population, combined with its rapidly advancing healthcare infrastructure, provides a fertile ground for clinical trials to flourish. This is an opportunity for top biopharma companies to develop a long-term strategy that focuses on the key enablers of innovation and strategic partnerships in India."
The reforms listed in the report include the creation of a hospital-based 'National Registry for Rare Diseases' by the Indian Council of Medical Research (ICMR) to overcome the dearth of epidemiological data for rare diseases in India. Besides, it also highlighted the launch of the National Biopharma Mission to strengthen the capacity to conduct clinical trials in India in the areas of Oncology, Ophthalmology, Rheumatology and Diabetology (CHOORD) across 5 speciality networks of 36 organizations (hospitals, research institutes) in 18 states.
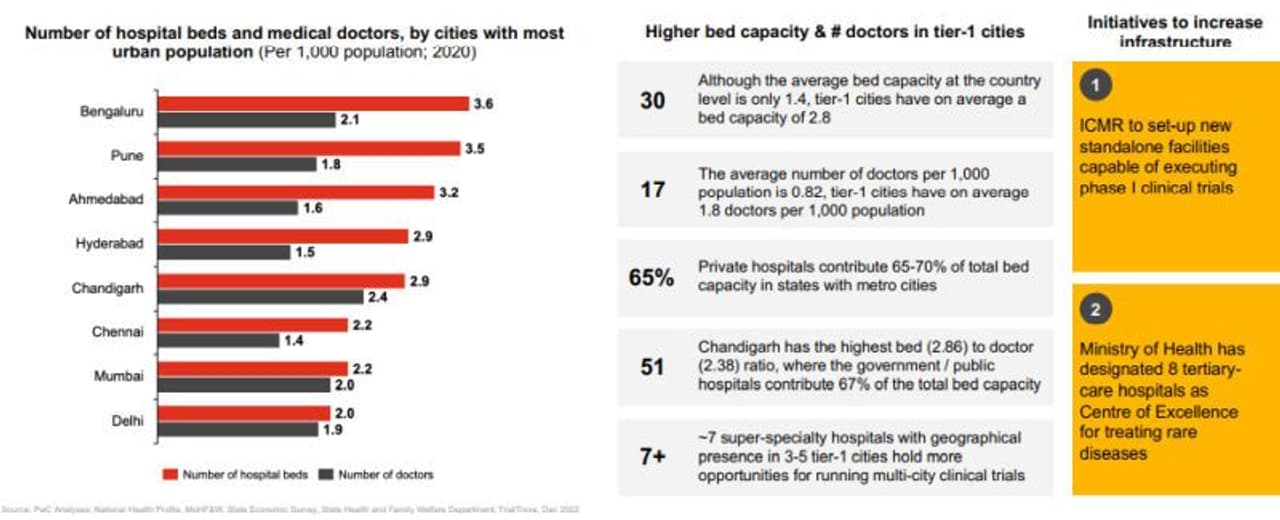
At the same time, the report noted the complexity of trial processes and the low trial participation rate that continue to exist in India. The report recommended top biopharma to align their strategy towards tier-1 cities (e.g., Mumbai, Delhi, Bengaluru, Chennai) where the higher bed capacity, number of doctors, and presence of tertiary care multi-city hospitals can support enablement efforts of running faster and more efficient clinical trials.
A significant number of Phase I–III clinical trials sponsored by big pharma with sites in Russia and Ukraine are still not recruiting participants. India is a potential site for reallocation of trials as the country has a high prevalence in the top disease areas where trial recruitment is halted, the report noted.
The report quoted Peyton Howell, Chief Operating and Growth Officer of Parexel, as saying: 'We are mitigating the risk [Russia-Ukraine situation]. We do think, though, it opens the door in terms of opportunities for India.'
Karun Rishi, President of USAIC, said: 'The growing interest in clinical trials in India presents a significant opportunity for private biopharma companies to leverage the country's rich diversity and robust healthcare infrastructure. With a large and diverse patient pool, streamlined regulatory processes, and a highly skilled workforce, India offers a favourable environment for biopharma companies to conduct efficient and cost-effective clinical trials. By tapping into this opportunity, companies can accelerate their drug development timelines, increase the efficiency of their research, and bring innovative treatments to patients in need, ultimately advancing global healthcare.'
