The FDA has now accepted the biologics license application for review, setting a Prescription Drug User Fee Act goal date of Aug. 5, 2026.
- Moderna has submitted a revised approach to streamline approval based on age-specific indications.
- The company proposed a dual-pathway regulatory strategy, seeking full FDA approval for adults aged 50 to 64 and accelerated approval for adults 65 and older.
- Last week, the FDA refused to review Moderna’s application for the flu vaccine.
Moderna, Inc. (MRNA) said on Wednesday that it is moving forward with regulatory review for its investigational seasonal flu vaccine, mRNA-1010, after responding to a prior Refusal-to-File letter from the U.S. Food and Drug Administration (FDA).

The company has submitted a revised approach to streamline approvals based on age-specific indications.
Targeted Approval Strategy
Moderna proposed a dual-pathway regulatory strategy, seeking full FDA approval for adults aged 50 to 64 and accelerated approval for adults aged 65 and older, along with a post-marketing study to further evaluate safety and efficacy in older populations.
The FDA has now accepted the biologics license application (BLA) for review, setting a Prescription Drug User Fee Act (PDUFA) goal date of Aug. 5, 2026.
"Pending FDA approval, we look forward to making our flu vaccine available later this year so that America's seniors have access to a new option to protect themselves against flu."
-Stéphane Bancel, CEO, Moderna
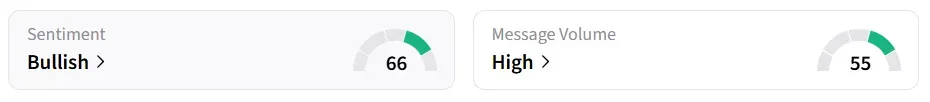
Following the update, Moderna’s stock traded over 5% higher on Wednesday morning. On Stocktwits, retail sentiment around the stock jumped to ‘bullish’ from ‘neutral’ territory the previous day amid ‘high’ message volume.
Global Regulatory Progress
mRNA-1010 has entered regulatory review not only in the U.S. but also in Europe, Canada, and Australia, with additional filings planned for later in 2026. Moderna anticipates the first potential approvals for the vaccine to occur within the year.
If approved, mRNA-1010 would be available for U.S. adults aged 50 and above, including those 65 and older, for the 2026/2027 influenza season.
Last week, the FDA refused to review Moderna’s application for the flu vaccine and cited the lack of an "adequate and well-controlled" study with a comparator arm that "does not reflect the best-available standard of care."
MRNA stock has gained over 28% in the last 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com.<
