FDA’s ZYCUBO approval also includes a Rare Pediatric Disease Priority Review Voucher, which will be transferred to Cyprium.
- The FDA’s green light comes after Sentynl Therapeutics, fully owned by Zydus Lifesciences, assumed responsibility for developing and commercializing CUTX-101.
- Cyprium stands to earn tiered royalties on ZYCUBO sales and up to $129 million in potential development and sales milestones from Sentynl.
- Children receiving early treatment with ZYCUBO had a nearly 80% lower risk of death compared with untreated patients.
Fortress Biotech, Inc. (FBIO) and its majority-owned unit, Cyprium Therapeutics, announced on Tuesday that the U.S. Food and Drug Administration (FDA) has officially approved ZYCUBO (formerly CUTX-101) for treating Menkes disease in children.

The FDA’s green light comes after Sentynl Therapeutics, fully owned by Zydus Lifesciences, assumed responsibility for developing and commercializing CUTX-101 from Cyprium in December 2023.
“The approval of ZYCUBO is a pivotal milestone for our company and patients suffering from Menkes Disease, as it is the first and only FDA-approved treatment for this rare, often fatal, pediatric disease.”
-Lindsay A. Rosenwald, Chairman, President and CEO, Fortress Biotech
Approval And Benefits
The approval also includes a Rare Pediatric Disease Priority Review Voucher, which will be transferred to Cyprium. Additionally, Cyprium stands to earn tiered royalties on ZYCUBO sales and up to $129 million in potential development and sales milestones from Sentynl.
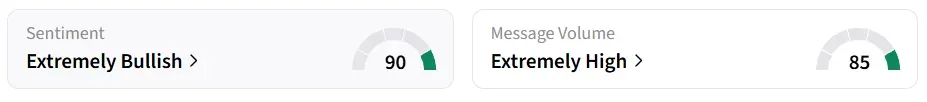
Following the update, Fortress Biotech stock traded over 12% higher in Tuesday’s premarket. On Stocktwits, retail sentiment around the stock improved to ‘extremely bullish’ from ‘bullish’ territory the previous day. Message volume changed to ‘extremely high’ from ‘high’ levels in 24 hours.
Menkes disease is a rare genetic disorder caused by mutations in the ATP7A gene, which disrupt copper absorption and transport in infants. Without treatment, the disease can be fatal, as copper is essential for brain development.
ZYCUBO Efficiency
ZYCUBO, administered as a subcutaneous injection, helps restore copper balance in the body and maintain adequate levels in patients with Menkes disease.
Results supporting approval showed that children receiving early treatment with ZYCUBO had a nearly 80% lower risk of death compared with untreated patients. Median overall survival in the treated group was 177.1 months, compared with 17.6 months in the external control group.
FBIO stock has gained over 113% in the last 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com.<
