Researchers uncover a crucial hidden function of the protein RPA, revealing how its failure to activate telomerase leads to dangerous telomere-related diseases. The discovery opens new diagnostic pathways for cancers and bone marrow disorders.
In a major scientific leap, researchers at the University of Wisconsin–Madison have uncovered a long overlooked function of a protein that helps explain why some people develop severe and sometimes fatal telomere-related diseases. The study shines new light on replication protein A (RPA), revealing its crucial and previously unconfirmed role in keeping our chromosomes stable.

Telomeres, the protective caps at the ends of chromosomes naturally shorten as we age. But when something goes wrong in the machinery that maintains them, telomeres can shrink too quickly, destabilizing DNA and triggering serious conditions such as certain cancers and bone marrow failure disorders. Until now, several of these mysterious cases had no known explanation.
RPA Emerges as a Key Player in Telomerase Activation
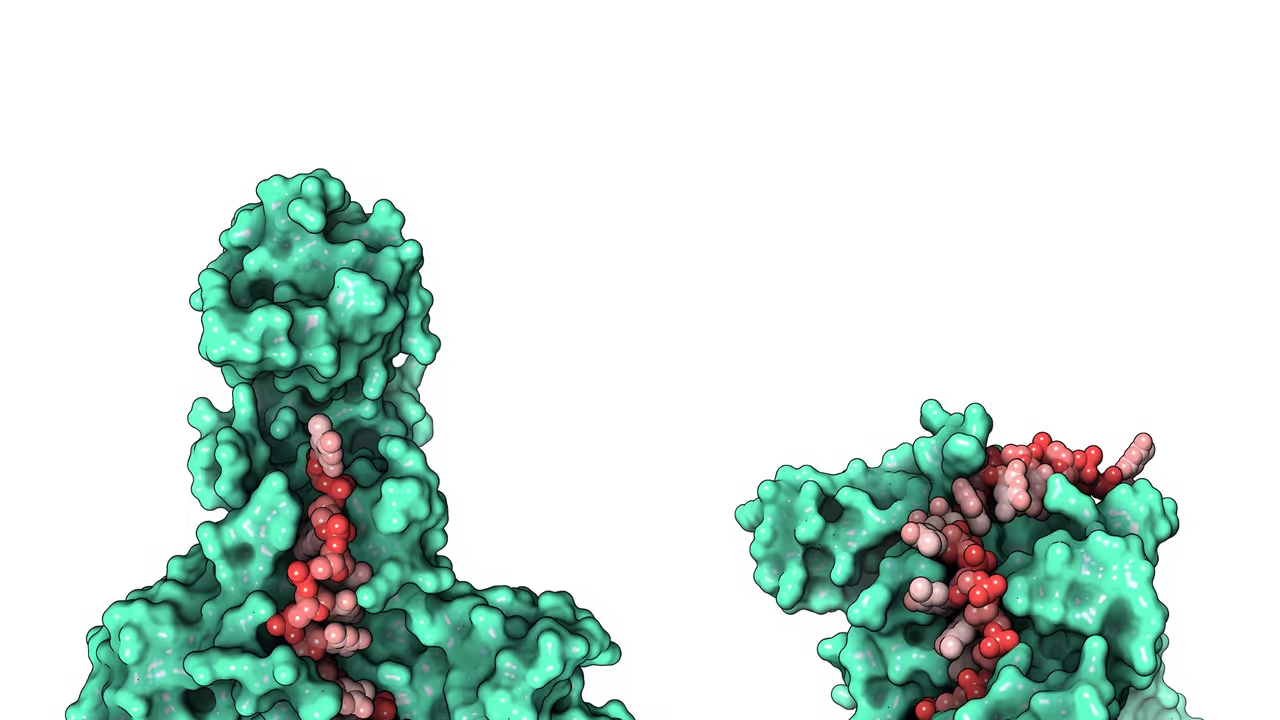
The research team, led by biochemist Ci Ji Lim, set out to identify proteins that work alongside telomerase, the enzyme that preserves telomere length. Using the powerful structural-prediction tool AlphaFold, graduate student Sourav Agrawal and colleagues pinpointed RPA as a likely partner.
RPA has long been recognized for its central role in DNA replication and repair, but its impact on telomere maintenance had never been confirmed in humans. The Wisconsin researchers validated that RPA directly stimulates telomerase, helping maintain healthy telomeres. When mutations disrupt RPA’s ability to activate telomerase, telomeres shorten abnormally — offering a compelling explanation for many previously puzzling patient cases.
New Diagnostic Possibilities for Life-Threatening Disorders
The findings provide a vital clue for clinicians treating patients with short-telomere conditions, including aplastic anemia, myelodysplastic syndrome and acute myeloid leukemia. For years, some patients showed hallmark signs of telomere dysfunction, yet genetic testing failed to reveal a cause. This discovery now fills a critical gap.
Following the study’s publication in Science, clinicians worldwide have contacted Lim’s team to investigate whether unexplained mutations in their patients may impair RPA’s newly identified role. By examining how these mutations affect RPA–telomerase interaction, doctors can finally offer families clearer answers and potentially more targeted care.
The discovery not only advances molecular biology but also brings new hope to patients whose conditions have long been shrouded in uncertainty.