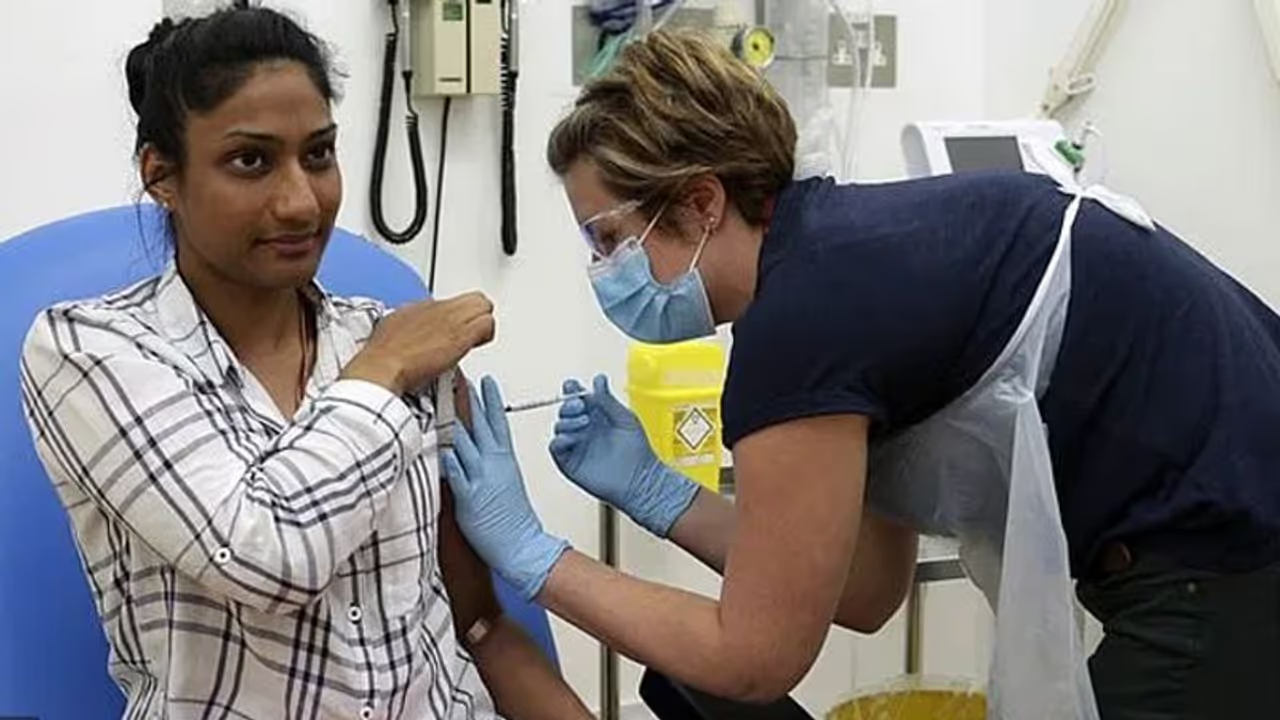
AIIMS-Delhi is among the 12 sites selected by the Indian Council for Medical Research (ICMR) for conducting phase I and II human trials of Covaxin
New Delhi: India's first coronavirus vaccine COVAXIN human trials are beginning today at the All India Institute of Medical Science (AIIMS), Delhi after its Ethics Committee gave its nod.

The hospital will start enrolling healthy individuals from today onwards.
"Few volunteers have already registered for the trial. We would start the screening of the individuals and evaluate their health condition from Monday onwards before vaccinating them," Dr Sanjay Rai, Professor at the Centre for Community Medicine at AIIMS had said.
AIIMS-Delhi is among the 12 sites selected by the Indian Council for Medical Research (ICMR) for conducting phase I and II human trials of Covaxin. In phase I, the vaccine would be tested on 375 volunteers and the maximum of 100 of them would be from AIIMS.
Covaxin is being jointly developed by Hyderabad-based Bharat Biotech in collaboration with the Indian Council of Medical Research (ICMR) and the National Institute of Virology (NIV). It recently received the nod for human clinical trials from the Drugs Controller General of India (DCGI).
Screenings to assess individuals' health conditions will start on Monday, Dr Sanjay Rai, Professor at the Centre for Community Medicine at AIIMS, has said.
"Few volunteers have already registered for the trial. We will start screening individuals and evaluate their health condition from Monday onwards before vaccinating them," he added.
"Healthy volunteers having no comorbid conditions and without a history of COVID-19, aged more than 18 years and less than 55 years, would be eligible to participate in a randomised, double-blind, placebo-controlled clinical trial," Dr Rai said.
Talking about the ethics committee's approval, he said it raised some objections concerning protocol initially, but they were addressed, following which the final nod to start human trials was granted.