The Data Monitoring Committee (DMC) recommended stopping the EMBOLD study ahead of schedule after the interim analysis demonstrated strong positive outcomes.
- Relutrigine targets patients suffering from severe, genetic epilepsy syndromes.
- To date, no approved therapy specifically addresses these conditions, making the trial’s success potentially important.
- Praxis will present the topline EMBOLD results on December 6 at the American Epilepsy Society’s meeting.
Praxis Precision Medicines, Inc. (PRAX) stock is on track to reach its January 2022 highs on Friday, after its experimental epilepsy drug candidate, relutrigine, produced positive results in the trials.

The Data Monitoring Committee (DMC) recommended stopping the study ahead of schedule after an interim analysis demonstrated strong positive outcomes.
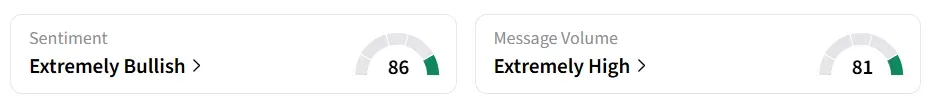
Praxis Precision’s stock traded over 24% higher in Friday’s premarket. On Stocktwits, retail sentiment around the stock improved to ‘extremely bullish’ from ‘bullish’ territory the previous day. Message volume jumped to ‘extremely high’ from ‘normal’ levels in 24 hours.
Why This Matters
Relutrigine targets patients suffering from severe, genetic epilepsy syndromes and developmental and epileptic encephalopathies (DEEs), neurological disorders marked by frequent seizures and high mortality.
To date, no approved therapy specifically addresses these conditions, making the trial’s success potentially important for affected children and families worldwide.
H.C. Wainwright boosted its price target for Praxis Precision to $340 from $258, citing that relutrigine delivered a “home run” in the EMBOLD trial. The firm also raised its estimated chance of the drug’s success to 80% from 60%, according to TheFly.
What Comes Next
Following the DMC’s recommendation, Praxis will present the topline EMBOLD results on December 6 at the American Epilepsy Society’s meeting in Atlanta. If regulatory discussions with the FDA go favorably, the company could soon file a New Drug Application (NDA) for relutrigine.
Basically, Relutrigine works as a preferential inhibitor of persistent sodium current, shown to be a key driver of seizure symptoms in severe DEEs.
Studies in live animal models show that relutrigine can reduce seizures in a dose-dependent way, even stopping them entirely in mice with SCN2A, SCN8A, and other DEE conditions. The drug has also been well-tolerated in three Phase 1 trials.
PRAX stock has gained over 146% in 2025 and over 178% in the last 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com.<