The stock is on track to record its best day since December 2024 if the overnight gains hold.
- The FDA approved Optune Pax for locally advanced pancreatic cancer.
- The Panova-3 trial showed Optune Pax, used alongside standard chemotherapy, improved median overall survival and delayed pain progression.
- Novocure recently reported preliminary Q4 revenue of $174.4 million and full-year 2025 revenue of $655.4 million, both ahead of consensus.
Shares of Novocure (NVCR) surged 30% in extended trading on Wednesday after the U.S. Food and Drug Administration (FDA) approved Optune Pax for the treatment of adults with locally advanced pancreatic cancer, marking the first FDA-approved therapy for this indication in almost three decades.

NVCR stock, which ended Wednesday’s regular session at $10.5, is on track to record its best day since December 2024 if the overnight gains hold.
Wearable Cancer Device Wins FDA Approval
Novocure said the FDA cleared Optune Pax based on results from the Phase 3 Panova-3 trial, which showed a statistically significant improvement in overall survival compared with chemotherapy alone.
The approval allows Optune Pax to be used together with standard chemotherapy drugs gemcitabine and nab-paclitaxel, which are commonly given to patients with locally advanced pancreatic cancer.
Optune Pax is a wearable, non-invasive medical device that delivers Tumor Treating Fields (TTFields), which are alternating electric fields that disrupt cancer cell division and survival without harming healthy cells. The device is worn continuously and targets tumors using a biophysical mechanism rather than systemic drugs.
“This approval has the potential to be practice changing,” said Vincent Picozzi, an investigator in the Panova-3 trial.
Phase 3 Trial Shows Survival And Pain Benefits
Panova-3 was an international, randomized Phase 3 trial that enrolled 571 patients with locally advanced pancreatic cancer.
In the main trial group, patients who received Optune Pax along with standard chemotherapy lived a median of 16.2 months, compared with 14.2 months for those treated with chemotherapy alone, representing an average survival benefit of about two months.
Among patients who remained on treatment longer, survival improved further. In this group, median survival reached 18.3 months, compared with 15.1 months for patients receiving chemotherapy alone, a difference of just over three months.
The study also showed that Optune Pax helped delay the worsening of cancer-related pain. Patients treated with the device went a median of 15.2 months before pain worsened, compared with 9.1 months for those on chemotherapy alone. Quality-of-life measures also showed that patients maintained better overall well-being and pain control for longer periods when Optune Pax was added to treatment.
Novocure Enters 2026 With Record Sales
Last month, H.C. Wainwright lowered its price target on Novocure to $39 from $42, while reiterating a ‘Buy’ rating. The firm said Novocure’s fourth-quarter (Q4) revenue growth highlighted continued momentum for its Optune Gio franchise, though it trimmed estimates related to lung cancer.
Novocure reported preliminary Q4 revenue of $174.4 million, topping consensus expectations of $168.93 million. The company also reported preliminary full-year 2025 revenue of $655.4 million, slightly ahead of consensus estimates.
CEO Frank Leonard said Novocure finished 2025 with record annual revenue, providing the financial strength to support multiple product launches in 2026 while remaining on a clear path toward profitability.
How Did Stocktwits Users React?
On Stocktwits, retail sentiment for NVCR was ‘extremely bullish’ amid a 475% surge in 24-hour message volume.
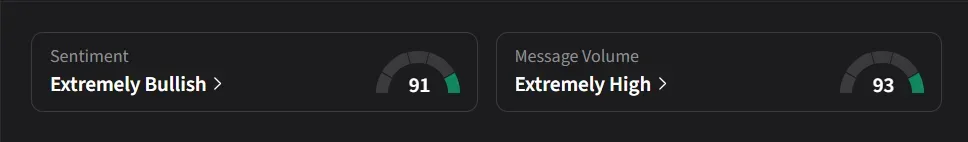
One user expects the stock to reach $22 soon.
Another user said the stock will “run huge tomorrow.”
NVCR stock has declined 51% over the past 12 months.
For updates and corrections, email newsroom[at]stocktwits[dot]com.<
