MIT researchers develop a revolutionary sodium-air fuel cell delivering over three times the energy density of lithium-ion batteries, enabling safer, carbon-negative electric aviation and transport.
In a landmark breakthrough that could reshape the future of electric transportation, engineers at the Massachusetts Institute of Technology (MIT) have developed a sodium-air fuel cell that delivers more than three times the energy density of current lithium-ion batteries. Lightweight, rechargeable, and carbon-negative, this technology holds game-changing potential for aviation, marine, and rail electrification.

Published in the journal Joule, the study presents a novel solution to the longstanding energy density limitations of modern batteries, which have slowed progress in electrifying heavier modes of transport like aircraft and cargo ships.
A Radical Alternative to Batteries: The Sodium-Air Fuel Cell
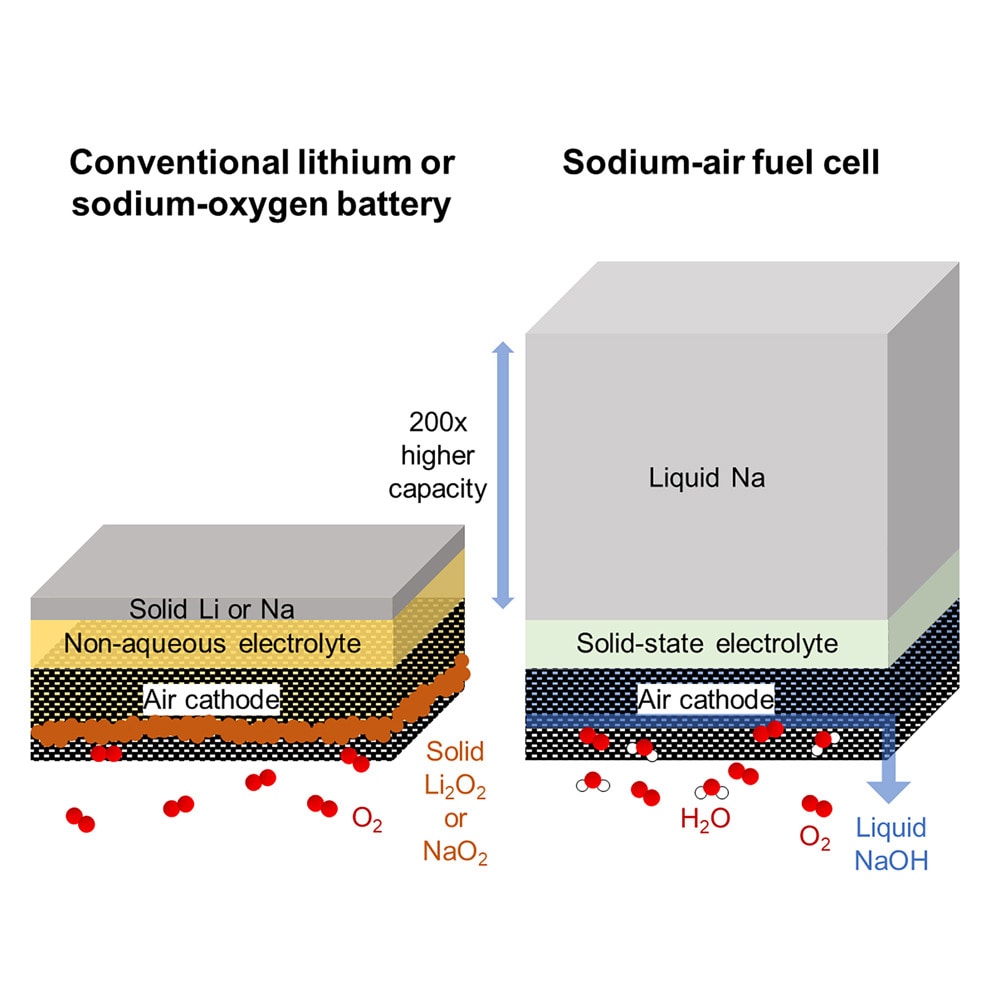
Instead of relying on lithium-ion chemistry, the MIT-led team has engineered a sodium-air fuel cell powered by a chemical reaction between liquid sodium metal and atmospheric oxygen. The core of this device features a solid ceramic electrolyte, flanked by liquid sodium on one side and a porous air electrode on the other. This setup enables the direct generation of electricity via sodium oxidation.
Unlike conventional batteries that require recharging, fuel cells can be refueled quickly. Sodium metal, the key fuel, is abundant, affordable, and historically produced at large scales, offering a sustainable and scalable alternative to lithium.
Game-Changing Energy Density for Electric Flight
During lab-scale tests, the sodium-air fuel cell prototype delivered nearly 1,700 watt-hours per kilogram at the individual stack level — translating to over 1,000 watt-hours per kilogram at the full system level.
That’s a critical milestone, says Professor Yet-Ming Chiang, senior author of the study and Kyocera Professor of Ceramics at MIT.
“The threshold that you really need for realistic electric aviation is about 1,000 watt-hours per kilogram,” Chiang explains. “Today’s electric vehicle lithium-ion batteries top out at about 300 watt-hours per kilogram — nowhere near what’s needed.”
While not yet sufficient for long-haul flights, this innovation could power regional electric aviation, which accounts for 80% of domestic flights and nearly 30% of aviation emissions.
Carbon-Negative Emissions: From Pollution to Baking Soda
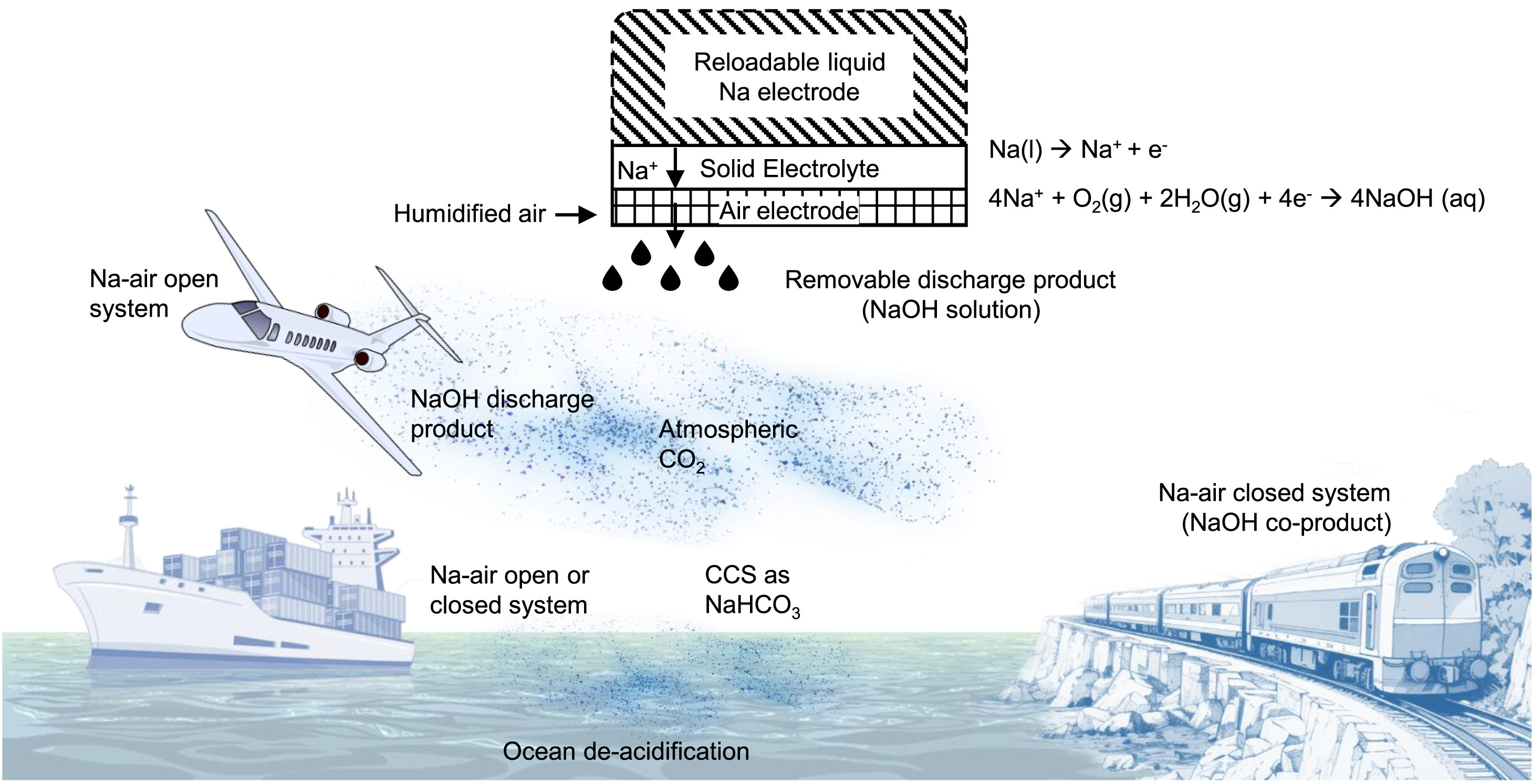
Perhaps most remarkably, the emissions from this sodium-air fuel cell actively reduce atmospheric carbon dioxide.
As sodium reacts with oxygen, it forms sodium oxide, which absorbs CO₂ to become sodium hydroxide. This compound quickly forms sodium carbonate, and ultimately sodium bicarbonate — commonly known as baking soda.
“There’s this natural cascade of reactions that happens when you start with sodium metal,” says Chiang. “It’s all spontaneous. We don’t have to do anything to make it happen, we just have to fly the airplane.”
If the byproduct ends up in the ocean, it could help de-acidify the water, offering an added environmental benefit. Chiang adds that sodium hydroxide has been proposed as a CO₂ mitigation solution, but has been too costly to produce — until now.
“But here, it’s a byproduct,” Chiang notes. “So it’s essentially free, producing environmental benefits at no cost.”
Safer by Design: Why a Fuel Cell Beats a Battery
Safety has long been a concern with high-energy-density storage systems, especially those involving reactive metals like sodium or lithium. But the MIT team argues that this fuel cell design is inherently safer than traditional batteries.
“Whenever you have a very high energy density battery, safety is always a concern,” Chiang explains. “If there’s a rupture of the membrane that separates the two reactants, you can have a runaway reaction. But in this fuel cell, one side is just air, which is dilute and limited. So you don’t have two concentrated reactants right next to each other. If you're pushing for really, really high energy density, you'd rather have a fuel cell than a battery for safety reasons.”
From Lab Bench to Skies: The Road to Commercialization
While the current prototype is small and lab-scale, researchers believe scaling up is straightforward. The team has already founded a startup, Propel Aero, to commercialize the technology. The company is currently incubated at MIT’s innovation hub, The Engine.
Their immediate goal is to develop a brick-sized cell capable of delivering 1,000 watt-hours of energy — enough to power a large agricultural drone. This demo unit is expected to be ready within a year.
“We expect people to think that this is a totally crazy idea,” says Chiang. “If they didn’t, I’d be a bit disappointed because if people don’t think something is totally crazy at first, it probably isn’t going to be that revolutionary.”
A Team Effort with Wide-Ranging Expertise
The fuel cell project is the result of interdisciplinary collaboration involving material science, fuel cell engineering, and high-temperature battery research.
“We’re pulling from fuel cell research in terms of designing our electrode, we’re pulling from older high-temperature battery research as well as some nascent sodium-air battery research, and kind of mushing it together,” says doctoral student Saahir Ganti-Agrawal. “That led to the big bump in performance.”
Another key insight, says doctoral student Karen Sugano, was the role of humidity in enabling liquid rather than solid discharge products — a critical factor for continuous operation and easier removal of byproducts.
“The key was that we can form this liquid discharge product and remove it easily, as opposed to the solid discharge that would form in dry conditions,” Sugano explains.
The project received support from ARPA-E, Breakthrough Energy Ventures, and the National Science Foundation, and utilized advanced fabrication facilities at MIT.nano.
Contributors also included researchers from Form Energy, And Battery Aero, and the University of Michigan, along with high school intern Alden Friesen from Arizona.
What’s Next?
With sodium being easy to extract from common salt (sodium chloride), and proven historical production capacity exceeding 200,000 tons a year in the US alone, MIT’s sodium-air fuel cell could become a viable and scalable solution for clean transportation.
If successful, the innovation promises to redefine electric propulsion for not just drones and planes, but also for trucks, trains, and ships — all while removing carbon from the atmosphere.


