Coronavirus news updates: 82-year-old gets Oxford vaccine shot in UK
The number of daily new Covid-19 cases in India remained below 20,000 for the third consecutive day. According to the data released by the government, there are 2,43,953 active cases of coronavirus infection in the country which comprise 2.36 per cent of the total caseload. Here are some other updates.
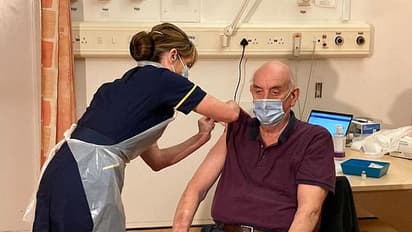
82-year-old receives AstraZeneca/Oxford vaccine shot
Brian Pinker, an 82-year-old dialysis patient, is the first patient to receive the AstraZeneca/Oxford vaccine.
The retired maintenance manager was vaccinated by nurse Sam Foster at Oxford's Churchill Hospital.
The health secretary described it as a "pivotal moment" in the UK's fight against the virus. More than half a million doses of the vaccine are ready for use on Monday.
82-year-old receives AstraZeneca/Oxford vaccine shot
Brian Pinker, an 82-year-old dialysis patient, is the first patient to receive the AstraZeneca/Oxford vaccine.
The retired maintenance manager was vaccinated by nurse Sam Foster at Oxford's Churchill Hospital.
The health secretary described it as a "pivotal moment" in the UK's fight against the virus. More than half a million doses of the vaccine are ready for use on Monday.
World's biggest inoculation drive set to begin: PM
Prime Minister Narendra Modi lauded scientists and technicians for the 'Made in India' vaccines and stated that the world's biggest inoculation drive against coronavirus is set to begin in the country.
PM Modi said, "World's biggest Covid-19 vaccination programme set to begin in India. For this, the country is proud of the contributions of its scientists and technicians."
"Quality is as much important as quantity, our standards should rise with our scale in our quest for Aatmanirbhar Bharat," he said.
World's biggest inoculation drive set to begin: PM
Prime Minister Narendra Modi lauded scientists and technicians for the 'Made in India' vaccines and stated that the world's biggest inoculation drive against coronavirus is set to begin in the country.
PM Modi said, "World's biggest Covid-19 vaccination programme set to begin in India. For this, the country is proud of the contributions of its scientists and technicians."
"Quality is as much important as quantity, our standards should rise with our scale in our quest for Aatmanirbhar Bharat," he said.
Tamil Nadu allows 100% occupancy in movie halls
Tamil Nadu government has given a go-ahead to increasing the seating capacity of cinemas, theatres, multiplexes to 100 per cent following COVID-19 protocols.
The decision follows a representation made by prominent Tamil actors and theatre owners associations requested that cinema halls be allowed to function at full capacity as new movies are scheduled to be released around the Pongal celebrations.
The government order states: "The seating capacity of Cinemas/theatres/Multiplexes shall be permitted to increase from existing 0% to 100% by following the Standard Operating Procedure issued already. Further, in order to create awareness among the spectators, the precautionary measures for Covid 19 shall also be screened during the showtime."
Tamil Nadu allows 100% occupancy in movie halls
Tamil Nadu government has given a go-ahead to increasing the seating capacity of cinemas, theatres, multiplexes to 100 per cent following COVID-19 protocols.
The decision follows a representation made by prominent Tamil actors and theatre owners associations requested that cinema halls be allowed to function at full capacity as new movies are scheduled to be released around the Pongal celebrations.
The government order states: "The seating capacity of Cinemas/theatres/Multiplexes shall be permitted to increase from existing 0% to 100% by following the Standard Operating Procedure issued already. Further, in order to create awareness among the spectators, the precautionary measures for Covid 19 shall also be screened during the showtime."
SII's Covishield to cost Rs 200 for Govt, Rs 1000 for open market
Serum Institute of India's head Adar Poonawalla has announced that the company's Covid-19 vaccine Covishield will be sold to the Indian government at Rs 200 per dose.
The same vaccine will be sold at a higher price of Rs 1,000 per dose in the open market.
In an interview to news agency Associated Press, Poonawalla said that the vaccines could be delivered to states within seven to 10 days of the company finalising a deal with the Indian government.
The Central Licensing Authority has granted permission to SII to manufacture for sale of Covidshield. The government also directed the company to submit the safety data, including that of Adverse Event Following Immunization, every 15 days for the first two months and monthly thereafter till completion of clinical trials.
SII's Covishield to cost Rs 200 for Govt, Rs 1000 for open market
Serum Institute of India's head Adar Poonawalla has announced that the company's Covid-19 vaccine Covishield will be sold to the Indian government at Rs 200 per dose.
The same vaccine will be sold at a higher price of Rs 1,000 per dose in the open market.
In an interview to news agency Associated Press, Poonawalla said that the vaccines could be delivered to states within seven to 10 days of the company finalising a deal with the Indian government.
The Central Licensing Authority has granted permission to SII to manufacture for sale of Covidshield. The government also directed the company to submit the safety data, including that of Adverse Event Following Immunization, every 15 days for the first two months and monthly thereafter till completion of clinical trials.
ZyCoV-D gets DCGI nod for phase 3 trials
The Drugs Controller General of India has approved Ahmedabad based drug firm Zydus Cadila to initiate Phase III clinical trials of its Covid-19 vaccine ZyCoV-D.
The Health Ministry, in a statement, said: "The Nation's first indigenously-developed DNA vaccine candidate against COVID-19, ZyCoV-D, by M/s Zydus Cadila has been approved by Drugs Controller General of India (DCGI), for the conduct of the Phase III clinical trials."
"Based on the recommendations of the Subject Expert Committee, which reviewed the interim data, the DCGI has accorded permission for conducting Phase-III clinical trial in 26,000 Indian participants," the Ministry said.
ZyCoV-D gets DCGI nod for phase 3 trials
The Drugs Controller General of India has approved Ahmedabad based drug firm Zydus Cadila to initiate Phase III clinical trials of its Covid-19 vaccine ZyCoV-D.
The Health Ministry, in a statement, said: "The Nation's first indigenously-developed DNA vaccine candidate against COVID-19, ZyCoV-D, by M/s Zydus Cadila has been approved by Drugs Controller General of India (DCGI), for the conduct of the Phase III clinical trials."
"Based on the recommendations of the Subject Expert Committee, which reviewed the interim data, the DCGI has accorded permission for conducting Phase-III clinical trial in 26,000 Indian participants," the Ministry said.
Scientists doubt Covaxin's efficacy
Questions are being raised by independent scientists with regard to the efficacy of the Bharat Biotech's Coronavirus vaccine Covaxin.
The questions arise from the fact that Bharat Biotech is yet to share the Phase 3 trial data.
Members of the National Covid-19 task force clarified that Covaxin would be used in a "clinical trial" mode. However, some scientists say that this raises doubts about the vaccine's efficacy.
Vaccine scientist and Christian Medical College professor, Dr Gagandeep Kang said: "If you take a look at what was proposed by the DCGI (Drug Controller General of India) as an adequate data package back in September when they published their draft guidance (for approval of Covid-19 vaccines), it was very clear that they wanted safety and efficacy data and it was expected that, for the safety data, there would be at least two months of follow up. In the case of Covaxin, you haven't finished enrolment. So, where is your safety data based on what DCGI asked for? Obviously, there's no efficacy data either."
Scientists doubt Covaxin's efficacy
Questions are being raised by independent scientists with regard to the efficacy of the Bharat Biotech's Coronavirus vaccine Covaxin.
The questions arise from the fact that Bharat Biotech is yet to share the Phase 3 trial data.
Members of the National Covid-19 task force clarified that Covaxin would be used in a "clinical trial" mode. However, some scientists say that this raises doubts about the vaccine's efficacy.
Vaccine scientist and Christian Medical College professor, Dr Gagandeep Kang said: "If you take a look at what was proposed by the DCGI (Drug Controller General of India) as an adequate data package back in September when they published their draft guidance (for approval of Covid-19 vaccines), it was very clear that they wanted safety and efficacy data and it was expected that, for the safety data, there would be at least two months of follow up. In the case of Covaxin, you haven't finished enrolment. So, where is your safety data based on what DCGI asked for? Obviously, there's no efficacy data either."