DCGI officially approves Serum and Bharat Biotech COVID-19 vaccine
The Drugs Controller General of India briefed the media at 11 am and officially approved emergency use authorisation for Serum and Bharat Biotech Covid-19 vaccines.
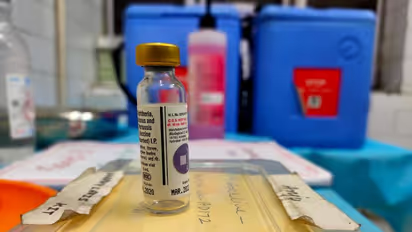
The Drugs Controller General of India briefed the media at 11 am and approved the emergency use authorisation for one of Serum and Bharat Biotech Covid-19 vaccines.
Serum Institute of India's Covishield and Bharat Biotech's Covaxin have been cleared by the subject expert committee (SEC) under the Central Drugs Standard Control Organisation for emergency use authorisation.
The expert panel had sent its recommendations to the DCGI chief VG Somani.
The Drugs Controller General of India briefed the media at 11 am and approved the emergency use authorisation for one of Serum and Bharat Biotech Covid-19 vaccines.
Serum Institute of India's Covishield and Bharat Biotech's Covaxin have been cleared by the subject expert committee (SEC) under the Central Drugs Standard Control Organisation for emergency use authorisation.
The expert panel had sent its recommendations to the DCGI chief VG Somani.
The Special Experts Committee, which reviewed Bharat Biotech's application on Covaxin, gave a favourable report to the Drugs Controller General of India on Saturday.
The vaccine: Covaxin is an indigenous inactivated two-dose SARS-CoV2 vaccine developed by Bharat Biotech along with the Indian Council of Medical Research and the National Institute of Virology. The vaccine has been developed and manufactured in Bharat Biotech's BSL-3 (Bio-Safety Level 3) bio-containment facility
Efficacy: According to Bharat Biotech, once the vaccine is injected into a human, it "has no potential to infect or replicate, since it is a killed virus". "It just serves to the immune system as a dead virus and mounts an antibody response towards the virus."
Storage: BBV152 or Covaxin is stored between 2 Degree Celsius and 8 Degree Celsius, which is compatible with all national immunization program cold chain requirements.
The Special Experts Committee, which reviewed Bharat Biotech's application on Covaxin, gave a favourable report to the Drugs Controller General of India on Saturday.
The vaccine: Covaxin is an indigenous inactivated two-dose SARS-CoV2 vaccine developed by Bharat Biotech along with the Indian Council of Medical Research and the National Institute of Virology. The vaccine has been developed and manufactured in Bharat Biotech's BSL-3 (Bio-Safety Level 3) bio-containment facility
Efficacy: According to Bharat Biotech, once the vaccine is injected into a human, it "has no potential to infect or replicate, since it is a killed virus". "It just serves to the immune system as a dead virus and mounts an antibody response towards the virus."
Storage: BBV152 or Covaxin is stored between 2 Degree Celsius and 8 Degree Celsius, which is compatible with all national immunization program cold chain requirements.
A day earlier, the government-appointed panel gave its go-ahead to Serum Institute of India's vaccine Covishield
The Vaccine: Covishield uses a replication-deficient chimpanzee viral vector based on a modified adenovirus virus (which causes the common cold) that contains genetic material from the SARS-CoV-2 virus. Vaccine dosage enables the immune system to attack the Covid-19 virus if it infects the body.
Cost: Serum Institute of India CEO Adar Poonawalla had earlier already stated that vaccine will cost $3 (Rs 219) per dose for the Indian government while but for the private market it will cost around Rs 700-800. Union Health Minister Harsh Vardhan has announced that the Covid-19 vaccine will be available free of cost across the country.
Storage: The Covishield vaccine can be stored, transported and handled at normal refrigerated conditions (two-eight degrees Celsius) for six months and administered within existing healthcare settings.
Stocks: SII has already manufactured 100 million doses of the vaccine, under the at-risk manufacturing and stockpiling license from DCGI. The stock will be enough to vaccinate 50 million people.
Efficacy: The primary efficacy based on a pooled analysis showed that the vaccine was 70.4 per cent (confidence interval: 54.8 per cent to 80.6 per cent) effective. Two doses administered with an interval of between four and 12 weeks showed clinical trials to be safe and effective at preventing symptomatic COVID-19, with no severe cases and no hospitalisations more than 14 days after the second dose.
A day earlier, the government-appointed panel gave its go-ahead to Serum Institute of India's vaccine Covishield
The Vaccine: Covishield uses a replication-deficient chimpanzee viral vector based on a modified adenovirus virus (which causes the common cold) that contains genetic material from the SARS-CoV-2 virus. Vaccine dosage enables the immune system to attack the Covid-19 virus if it infects the body.
Cost: Serum Institute of India CEO Adar Poonawalla had earlier already stated that vaccine will cost $3 (Rs 219) per dose for the Indian government while but for the private market it will cost around Rs 700-800. Union Health Minister Harsh Vardhan has announced that the Covid-19 vaccine will be available free of cost across the country.
Storage: The Covishield vaccine can be stored, transported and handled at normal refrigerated conditions (two-eight degrees Celsius) for six months and administered within existing healthcare settings.
Stocks: SII has already manufactured 100 million doses of the vaccine, under the at-risk manufacturing and stockpiling license from DCGI. The stock will be enough to vaccinate 50 million people.
Efficacy: The primary efficacy based on a pooled analysis showed that the vaccine was 70.4 per cent (confidence interval: 54.8 per cent to 80.6 per cent) effective. Two doses administered with an interval of between four and 12 weeks showed clinical trials to be safe and effective at preventing symptomatic COVID-19, with no severe cases and no hospitalisations more than 14 days after the second dose.