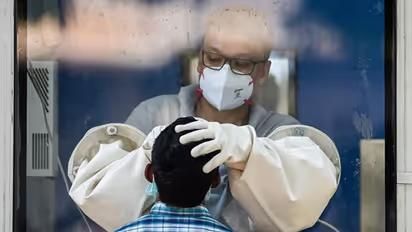
Coronavirus: Vital parameters of Oxford COVID-19 vaccine trial volunteers normal, says doctor
Published : Aug 27, 2020, 12:08 PM IST
COVID-19 vaccine developed by the University of Oxford started it’s phase II human trials in India on Wednesday (August 26)
Stay updated with the Breaking News Today and Latest News from across India and around the world. Get real-time updates, in-depth analysis, and comprehensive coverage of India News, World News, Indian Defence News, Kerala News, and Karnataka News. From politics to current affairs, follow every major story as it unfolds. Get real-time updates from IMD on major cities weather forecasts, including Rain alerts, Cyclone warnings, and temperature trends. Download the Asianet News Official App from the Android Play Store and iPhone App Store for accurate and timely news updates anytime, anywhere.