The 2-DG comes in powder form in a sachet, taken orally by dissolving it in water. It accumulates in the virus-infected cells and prevents virus growth.
The Drugs Controller General of India has approved the emergency use of a drug against Covid-19 manufactured by a lab under the Defence Research and Development Organisation.

The drug, 2-deoxy-D-glucose or 2-DG, was developed by the Institute of Nuclear Medicine and Allied Sciences in collaboration with the Hyderabad-based Dr Reddy's Laboratories.
The 2-DG comes in powder form in a sachet, taken orally by dissolving it in water. It accumulates in the virus-infected cells and prevents virus growth.
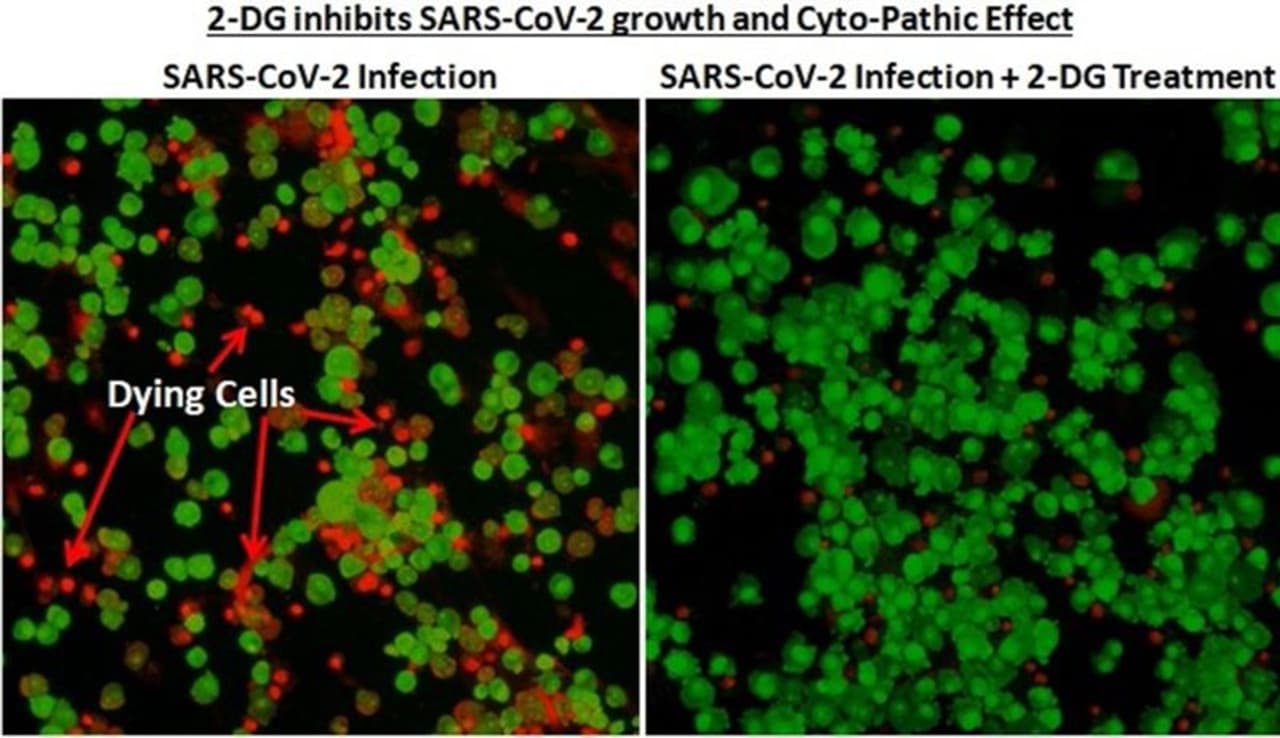
A significant number of patients treated with 2-DG showed RT-PCR negative conversion in Covid patients. The drug can help the infected patients to fight against the virus.
INMAS-DRDO scientists conducted laboratory experiments in April 2020 with assistance from the Hyderabad-based Centre for Cellular and Molecular Biology. They found that the drug worked effectively against the SARS-CoV-2 virus and restrained viral growth.
After that, the CDSCO permitted Phase-II clinical trial of the 2-DG in May 2020. DRDO and Dr Reddy's Lab started the clinical trial on 110 Covid-19 patients to test the drug's efficacy. The drug, during the next months in 16 hospitals, was found to be effective against the virus as the patient's health improved significantly. Patients treated with 2-DG showed faster symptomatic cure in efficacy trends than standard care patients.
According to DRDO, in December 2020, phase 3 trials on the drug began, during which 220 patients were given the drug in 27 Covid hospitals across nine states and New Delhi. The phase 3 data showed a significant number of patients administered with the drug came off supplemental oxygen dependence (42% vs 31%) by Day-3 compared to standard care patients.
Being a generic molecule, the drug can easily be produced, and in a short period, it can be distributed across the country.
NOTE: Asianet News humbly requests everyone to wear masks, sanitize, maintain social distancing and get vaccinated as soon as eligible. Together we can and will break the chain #ANCares #IndiaFightsCorona
